NUANCE researchers study potential advantages of sodium-ion batteries
A research team in Northwestern University's NUANCE Center is studying the sodium-ion battery and how it compares against the lithium-ion battery.
According to Dr. Jinsung Wu, research assistant professor at Northwestern University, "the sodium-ion battery has many potential advantages to the lithium-ion battery, as sodium being a low cost and environmentally benign element," Wu said. In the current study, the reaction mechanism of sodium with Co3O4 and carbon nanotube composite has been studied and revealed by in-situ transmission electron microscope. This finding provides further insights on the reaction mechanism in systems where many components are integrated and guidance in developing high quality nanocomposites as electrodes for sodium-ion batteries."
ABSTRACT:
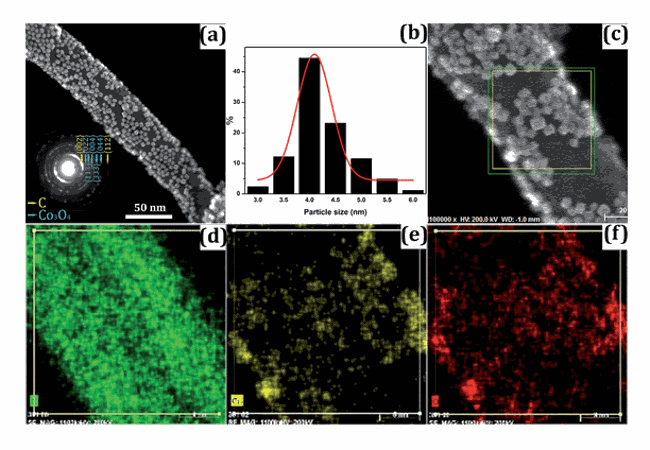
Replacing lithium with sodium-ion batteries for energy storage is of enormous interest, especially from practical and economic considerations. However, it has proved difficult to achieve competitive figures of merit for sodium-ion batteries due to the lack of a detailed understanding of the reaction mechanism(s). Herein, we report a sodium electrochemical conversion reaction with Co3O4 nanoparticles decorated on carbon nanotubes (Co3O4/CNTs) utilizing in situ TEM, down to the atomic-scale. We observe synergetic effects of the two nanoscale components, which provide insights into a new sodiation mechanism, facilitated by Na-diffusion along a CNT backbone and CNT–Co3O4 interfaces. A thin layer of amorphous low conductivity Na2O forms on the CNT surfaces at the beginning of sodiation. The conversion reaction results in the formation of ultrafine metallic Co nanoparticles and polycrystalline Na2O, and fast diffusion of the reaction products which might be due to the quick migration of Na2O under an electron beam. In the desodiation process, the dissociation of Na2O and formation of Co3O4 due to the de-conversion reaction are observed.
Qianqian Li, Jinsong Wu, Junming Xu and Vinayak P. Dravid. J. Mater. Chem. A, 2016,4, 8669-8675.
Full Article: http://pubs.rsc.org/en/content/articlehtml/2016/ta/c6ta02051h
